How to Determine Hybridization
How to Determine Hybridization
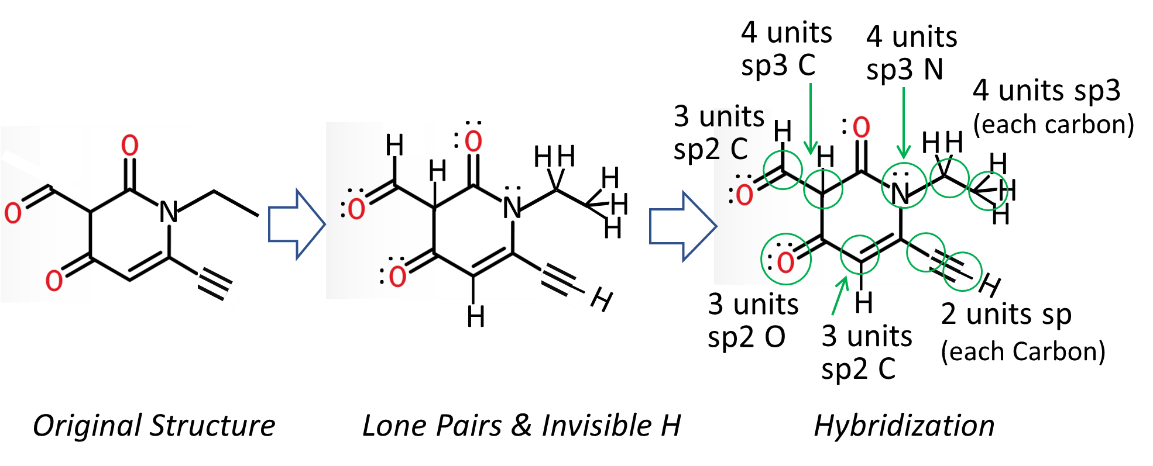
Hybridization answers the question of how many groups are associated with a particular atom in a structure. For example, if you were asked how many tires are associated with an automobile, the answer would be 4. On the other hand, the number of tires associated with a dirt bike would be 2, and those associated with a child’s tricycle would be 3. So it is for hybridization. Hybridization takes the count of the associated atoms (attachments) plus the number of lone pairs and arranges them into a geometrical system of s and p orbitals.
Put simply, it counts the objects around the atom and places 1 in the s location and up to 3 others in the p location. Note that the s will also only be one, but it’s invisible. And the good news for most organic compounds is that you only need up to 4 total locations. Four total locations would result in sp3 hybridization. And that’s it! Before we try it, here are some helpful tips. These tips assume that carbon does not have a positive (+) or negative (-) charge. This topic is discussed elsewhere.
The first tip is on how lone pairs, double bonds, and triple bonds should be counted.
- Count each set of lone pairs only as 1 object, not as individual dots. That means that two sets of lone pairs constitute 2 objects, not four.
- Count double bonds only as 1 object, not as individual bonds. That means that one double bond leads to 1 object.
- Count triple bonds only as 1 object, not as individual bonds. That means that one triple bond leads to 1 object.
So what if you are having a hard time counting the objects? Try the following:
- Add in the invisible lone pairs first. If they are already there, skip this step. Invisible lone pairs are commonly needed for oxygen (O) and nitrogen (N).
- Oxygen: O will have3 sets of lone pairs if it has 1 bond, 2 sets if it has 2 bonds, and 1 set if it has 3 bonds.
- Nitrogen: N will have 1 set of lone pairs if it has 3 bonds, none if it has 4 bonds (but it will have a plus (+) charge with 4 bonds)
2. Add in the invisible H atoms. Note that in skeletal structures, there is 1 H atom at blank double bonds, there are 2 H atoms at each blank bend, and 3 H atoms at each blank tip. There is also an H at the end of every triple bond and every aldehyde.
3. Draw a ring about the carbon or nitrogen or oxygen you are interested in. Carbon is the common target in organic chemistry.
4. Count the directions or objects coming from that carbon like it’s the intersection of a city street. The minimal number is 2.
5. Remember that always accounts for 1 and up to 3 will be assigned to p. The minimal hybridization is sp, which has only two objects.
Try the process by yourself. In general:
- sp3 = 4 single bonds to C, 3 single bonds to N, 2 or 1 single bonds to O
- sp2 = 1 double bond to C, N,or O
- sp = 1 triple bond to C, O, or N